Imagine living in a world where you couldn’t say if it is hot or cold, what the time is, how much you weigh or how long you’ve walked to reach a destination. This might sound impossible, but it is not far away from the everyday experience of people before the 1790s, when for the very first time, people realized the need of standardized units to measure length, time, weight and temperature. So they came up with the metric system in 1795, which evolved to the International System of Units (SI) in 1960. The world could now accurately measure in metres, seconds, kilograms and the kelvin.
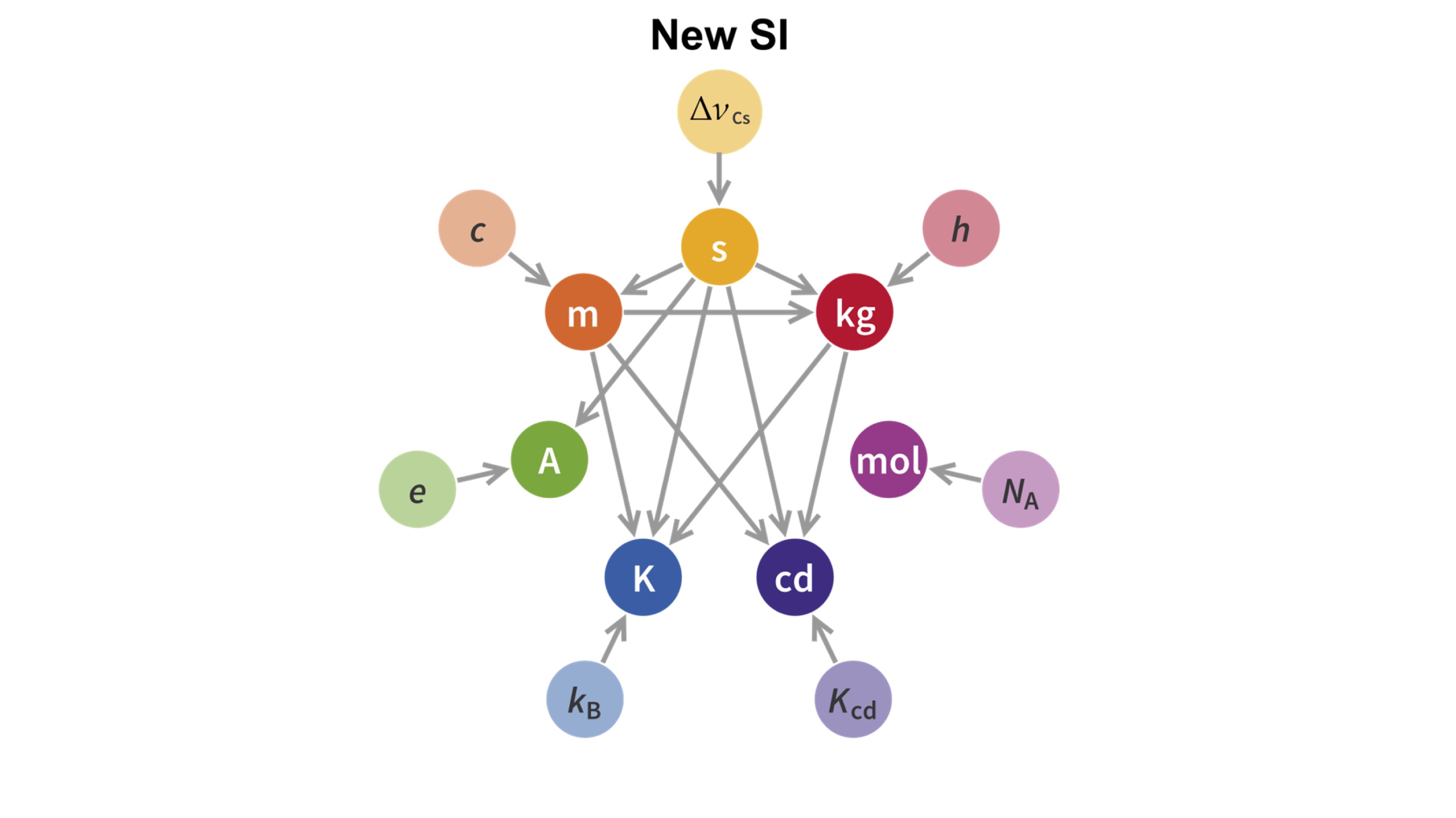
However, they all remained units defined in a random way; there is not much difference when you define a metre as the length of a random metal bar or as the forearm of the Pharaoh, as they did in ancient Egypt. As science and technology progressed, it became evident that standarisation should be linked to fundamental constants of nature. This is why length is linked with the speed of light, time is linked with the properties of atomic structure and the candela, the unit of luminous intensity, is linked with monochromatic radiation. These were the three first SI units to be properly linked with natural constants, and now, the rest are to follow.
The final units fall into line
A momentous occasion in the world of measurement science will happen on World Metrology Day (20thMay), to coincide with the 144thanniversary of the Metre Convention. The kilogram, ampere, mole and kelvin will join the other three SI units (the second, the candela and the metre) in having their definitions linked to fundamental constants of nature. Consigning the remaining artefacts such as the Grand K, the prototype kilogram kept near Paris and used as the global standard for the exact mass of the kilogram, to the history books.
Scientists ensure that we will not burn the cakes
Academics at Royal Holloway, University of London were instrumental in the adoption of the new definition of the kelvin, the unit that measures temperature. Scientists use the kelvin to describe both the hottest objects in the universe and the coldest experiments in laboratories close to absolute zero. Since 1954 the definition of the kelvin relied upon the triple point of water - a precise temperature where water exists in all three states of matter: solid, liquid and gas in equilibrium, 273.16 K or 0.01 °C. This has now been replaced with a definition that links temperature to its equivalent energy through the Boltzmann constant.
We all know that hot air balloons fly. The air inside the balloon is given more energy, which means that on average the molecules are moving faster and hence take up more volume in a given time, making the balloon expand. The relationship between the average kinetic energy of particles in a gas with the temperature of the gas is described by the Boltzmann constant, named after the physicist Ludwig Boltzmann.
In 1983 the speed of light was assigned an exact numerical value of 299,792,458 m/s, fixing the definition of the metre. Now following a decade of careful measurements at national standards laboratories, like NPL in the UK, the Boltzmann constant has undergone the same treatment, which fixes the definition of the kelvin.
The redefinition will not change the value of the units as we know them, but will ensure that as technologies evolve we can make better measurements in the future and provide a more coherent framework showing the relationship between the units.
How easy it is to accurately define temperature?
Linking a unit to a fundamental constant requires lots of data to minimize the uncertainties in the measurements. Professor John Saunders, Dr Andrew Casey, Dr Lev Levitin and Dr Chris Lusher, all from the Department of Physics at Royal Holloway, worked on the new definition of the kelvin for six years, developing an ideal thermometer for testing and confirming the new scale. The project was in partnership with the European National Measurement Institutes National Physical Laboratory (UK), Physikalisch-Technische Bundesanstalt (Germany) and MIKES (Finland), funded through The European Metrology Programme for Innovation and Research.
Dr Andrew Casey said: “We’re very excited about this new definition for the kelvin and hope that the new thermometers developed will ensure that the exact definition can be realized in laboratories around the world. The inclusion of our thermometer in the Mise en Pratique provides the framework for disseminating the new kelvin”
An absolute thermometer for ultra-low temperatures
“At Royal Holloway we developed a thermometer, called a Current Sensing Noise Thermometer, that measures the thermal noise of a low temperature material. As you get colder the motion of the particles that cause the noise decreases, directly related to the Boltzmann constant, so you need a really sensitive device to detect it. The group at Royal Holloway are experts at using superconducting devices called SQUIDs (Superconducting Quantum Interference Device) to measure really small signals. Using a SQUID to readout their thermometer allowed direct measurement of the property linked to the Boltzmann constant and so was ideal for confirming the new scale at the lowest temperatures.”